Oct 31, 2023menu_book Bookshelves perm_media Learning Objects login Login how_to_reg Request Instructor Account hub Instructor Commons Search Submit Search Downloads expand_more Download Page (PDF) Download Full Book (PDF) Resources expand_more Periodic Table Physics Constants Scientific Calculator Reference expand_more Reference & Cite Tools expand_more
If semipermeable membrane is placed between the solvent and solution as shown in the given figure then – Sarthaks eConnect | Largest Online Education Community
concept Osmosis Concept 1 1m 0 Comments Mark as completed Was this helpful? 1 2 concept Osmosis Concept 2 2m 0 Comments Mark as completed Was this helpful? 0 3 example Osmosis Example 1 38s 0 Comments Mark as completed Was this helpful? 0 4 Problem A semipermeable membrane is placed between the following solutions.

Source Image: studypool.com
Download Image
Tonicity is a bit different from osmolarity because it takes into account both relative solute concentrations and the cell membrane’s permeability to those solutes. Three terms—hypertonic, hypotonic, and isotonic—are used to describe whether a solution will cause water to move into or out of a cell: If a cell is placed in a hypertonic

Source Image: discoveryexpresskids.com
Download Image
A semipermeable membrane is placed between the following solution… | Channels for Pearson+ Sep 16, 2022Figure 13.10. 1: Osmosis. (a) Two solutions of differing concentrations are placed on either side of a semipermeable membrane. (b) When osmosis occurs, solvent molecules selectively pass through the membrane from the dilute solution to the concentrated solution, diluting it until the two concentrations are the same.
Source Image: numerade.com
Download Image
A Semipermeable Membrane Is Placed Between The Following Solutions
Sep 16, 2022Figure 13.10. 1: Osmosis. (a) Two solutions of differing concentrations are placed on either side of a semipermeable membrane. (b) When osmosis occurs, solvent molecules selectively pass through the membrane from the dilute solution to the concentrated solution, diluting it until the two concentrations are the same. A semipermeable membrane is placed between the following solutions. Which solution will increase in volume? A. Solution A:6.78% NaCl. B.
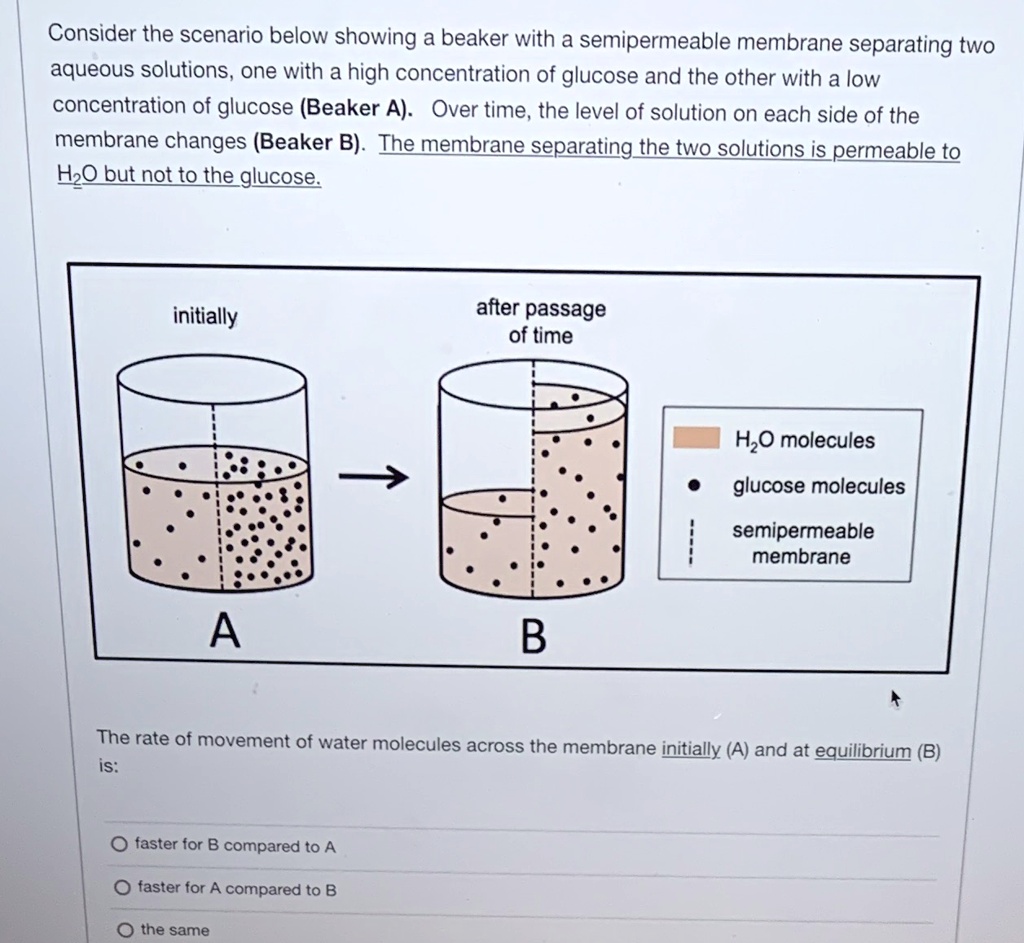
SOLVED: Consider the scenario below showing a beaker with a semipermeable membrane separating two aqueous solutions, one with a high concentration of glucose and the other with a low concentration of glucose (
Reviewed by: BD Editors Last Updated: October 4, 2019 Semipermeable Membrane Definition A semipermeable membrane is a layer that only certain molecules can pass through. Semipermeable membranes can be both biological and artificial. Give an example of a semipermeable membrane.

Source Image: byjus.com
Download Image
Semipermeable Membrane | Definition, Function & Examples – Video & Lesson Transcript | Study.com Reviewed by: BD Editors Last Updated: October 4, 2019 Semipermeable Membrane Definition A semipermeable membrane is a layer that only certain molecules can pass through. Semipermeable membranes can be both biological and artificial.

Source Image: study.com
Download Image
If semipermeable membrane is placed between the solvent and solution as shown in the given figure then – Sarthaks eConnect | Largest Online Education Community Oct 31, 2023menu_book Bookshelves perm_media Learning Objects login Login how_to_reg Request Instructor Account hub Instructor Commons Search Submit Search Downloads expand_more Download Page (PDF) Download Full Book (PDF) Resources expand_more Periodic Table Physics Constants Scientific Calculator Reference expand_more Reference & Cite Tools expand_more

Source Image: sarthaks.com
Download Image
A semipermeable membrane is placed between the following solution… | Channels for Pearson+ Tonicity is a bit different from osmolarity because it takes into account both relative solute concentrations and the cell membrane’s permeability to those solutes. Three terms—hypertonic, hypotonic, and isotonic—are used to describe whether a solution will cause water to move into or out of a cell: If a cell is placed in a hypertonic

Source Image: pearson.com
Download Image
If two solutions of 5 % glucose and 10 % glucose are kept in a trough seperated by a semipermeable membrane what will you observe after an hour? See Answer Question: A semipermeable membrane is placed between the following solutions. Which solution will decrease in volume? Solution A: 2.79 % (m/v) starch Solution B: 7.2 % (m/v) starch A semipermeable membrane is placed between the following solutions. Which solution will decrease in volume? Solution A: 2.79 % (m/v) starch

Source Image: byjus.com
Download Image
Selectively Permeable Membranes – Activity – TeachEngineering Sep 16, 2022Figure 13.10. 1: Osmosis. (a) Two solutions of differing concentrations are placed on either side of a semipermeable membrane. (b) When osmosis occurs, solvent molecules selectively pass through the membrane from the dilute solution to the concentrated solution, diluting it until the two concentrations are the same.

Source Image: teachengineering.org
Download Image
Egg Osmosis Lab Results – Bing Images | Osmosis, Osmosis jones, Biology lessons A semipermeable membrane is placed between the following solutions. Which solution will increase in volume? A. Solution A:6.78% NaCl. B.

Source Image: pinterest.com
Download Image
Semipermeable Membrane | Definition, Function & Examples – Video & Lesson Transcript | Study.com
Egg Osmosis Lab Results – Bing Images | Osmosis, Osmosis jones, Biology lessons concept Osmosis Concept 1 1m 0 Comments Mark as completed Was this helpful? 1 2 concept Osmosis Concept 2 2m 0 Comments Mark as completed Was this helpful? 0 3 example Osmosis Example 1 38s 0 Comments Mark as completed Was this helpful? 0 4 Problem A semipermeable membrane is placed between the following solutions.
A semipermeable membrane is placed between the following solution… | Channels for Pearson+ Selectively Permeable Membranes – Activity – TeachEngineering See Answer Question: A semipermeable membrane is placed between the following solutions. Which solution will decrease in volume? Solution A: 2.79 % (m/v) starch Solution B: 7.2 % (m/v) starch A semipermeable membrane is placed between the following solutions. Which solution will decrease in volume? Solution A: 2.79 % (m/v) starch