Stable Isotopes Electrons and Electron Configuration The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Beryllium is 4.
Alt AS CHM 02 Electronic Configuration Notes | PDF | Proton | Neutron
Electron affinity The energy released when an electron is added to the neutral atom and a negative ion is formed. Electronegativity (Pauling scale) The tendency of an atom to attract electrons towards itself, expressed on a relative scale. First ionisation energy The minimum energy required to remove an electron from a neutral atom in its

Source Image: geeksforgeeks.org
Download Image
C We obtain the valence electron configuration by ignoring the inner orbitals, which for phosphorus means that we ignore the [Ne] closed shell. This gives a valence-electron configuration of 3 s2 3 p3. Exercise 6.8.1 6.8. 1. Draw an orbital diagram and use it to derive the electron configuration of chlorine, Z = 17.

Source Image: pinterest.com
Download Image
Answered: Draw the electron configuration for a… | bartleby
An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. This is sometimes called the Bohr, or the ‘solar system’, model. Download this

Source Image: byjus.com
Download Image
Draw The Electron Configuration For A Neutral Atom Of Beryllium.
An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. This is sometimes called the Bohr, or the ‘solar system’, model. Download this
The way to use this is to first draw the table, which should be pretty easy to remember because the row numbers correspond to energy levels and the columns correspond to orbital types. … helium, lithium, and beryllium, look like. 1 s 1 1 s 2 1 s 2 2 s 1 1 s 2 2 s 2 and so on for the remaining elements. When chemists write down
Electronic Configuration of First 30 Elements with Atomic Numbers | BYJU’S
Chemistry Chemistry questions and answers O ELECTRONIC STRUCTURE Drawing a box diagram of the electron configuration of an atom Draw the electron configuration for a neutral atom of beryllium. energy ? This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer
Four possible electron configurations for a nitrogen atom are sho… | Channels for Pearson+

Source Image: pearson.com
Download Image
Beryllium (Be). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of beryllium-9 (atomic numb Stock Photo – Alamy
Chemistry Chemistry questions and answers O ELECTRONIC STRUCTURE Drawing a box diagram of the electron configuration of an atom Draw the electron configuration for a neutral atom of beryllium. energy ? This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer
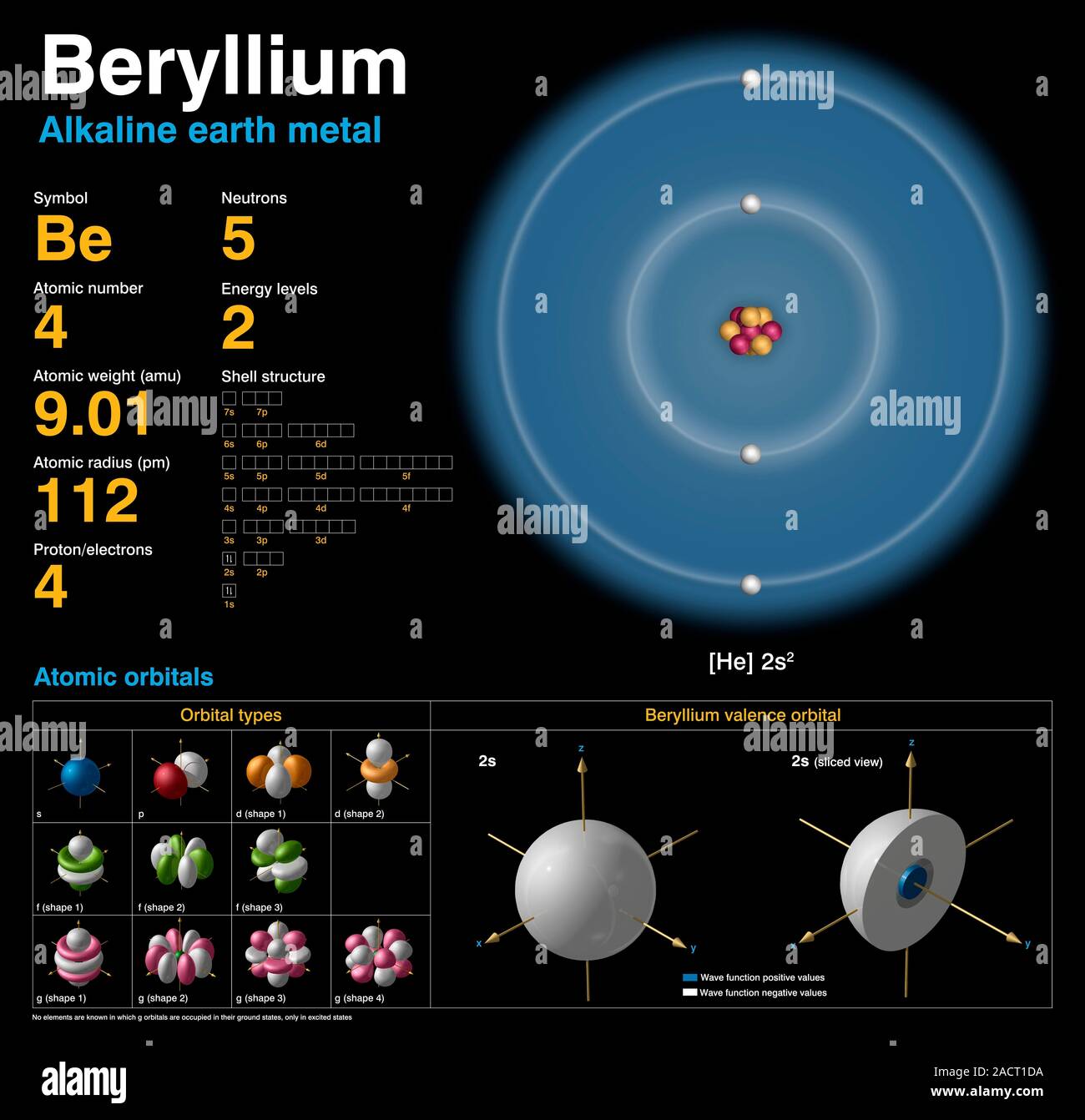
Source Image: alamy.com
Download Image
Alt AS CHM 02 Electronic Configuration Notes | PDF | Proton | Neutron
Stable Isotopes Electrons and Electron Configuration The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Beryllium is 4.

Source Image: scribd.com
Download Image
Answered: Draw the electron configuration for a… | bartleby
C We obtain the valence electron configuration by ignoring the inner orbitals, which for phosphorus means that we ignore the [Ne] closed shell. This gives a valence-electron configuration of 3 s2 3 p3. Exercise 6.8.1 6.8. 1. Draw an orbital diagram and use it to derive the electron configuration of chlorine, Z = 17.

Source Image: bartleby.com
Download Image
Solved) – Give the ground state electron configuration for Se.. Give the… (1 Answer) | Transtutors
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ).

Source Image: transtutors.com
Download Image
Electronic Configuration Of Neutral Beryllium He 2s2 Royalty Free SVG, Cliparts, Vectors, and Stock Illustration. Image 190384831.
An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. This is sometimes called the Bohr, or the ‘solar system’, model. Download this
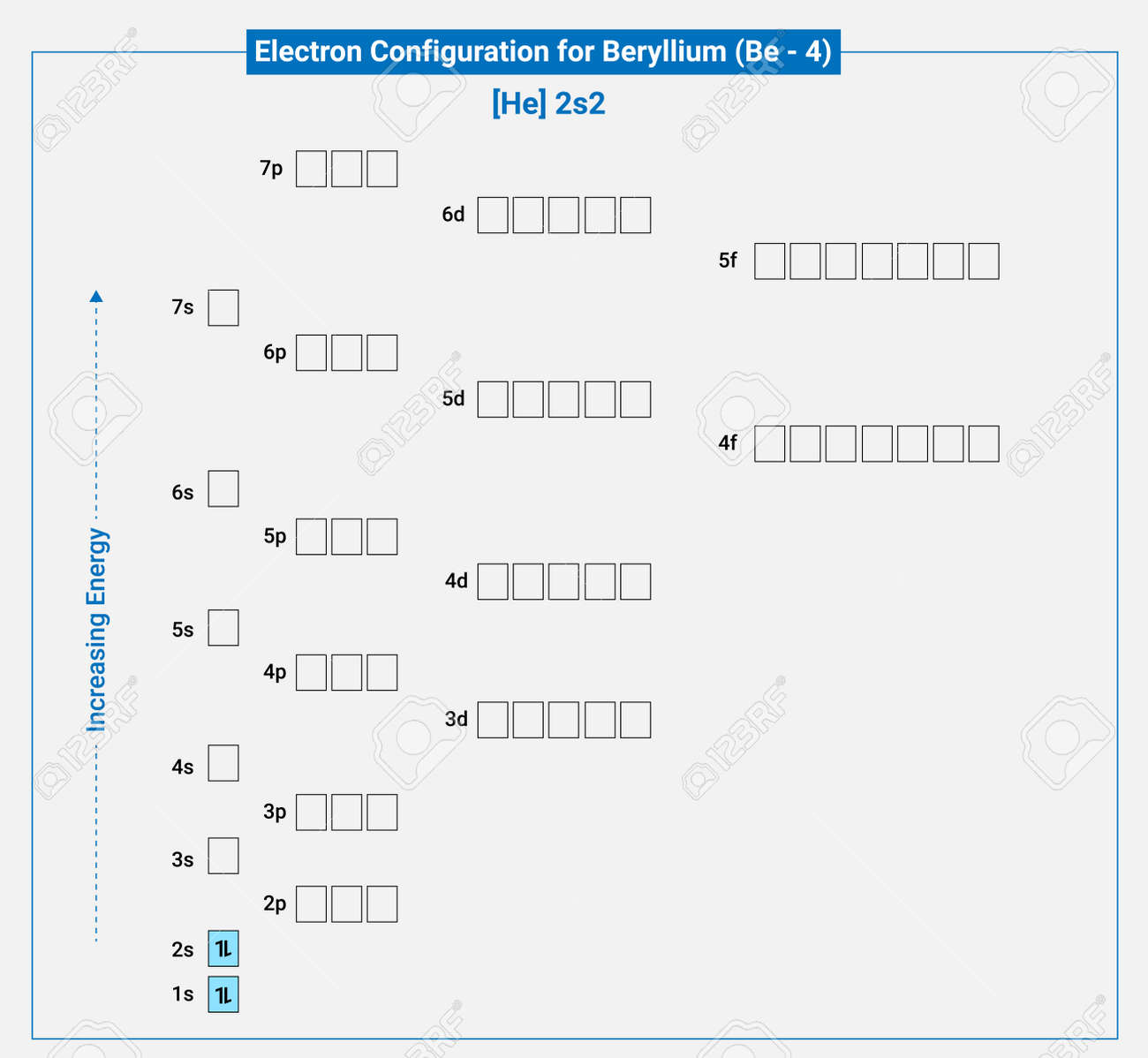
Source Image: 123rf.com
Download Image
Answered: Draw the electron configuration for a… | bartleby
The way to use this is to first draw the table, which should be pretty easy to remember because the row numbers correspond to energy levels and the columns correspond to orbital types. … helium, lithium, and beryllium, look like. 1 s 1 1 s 2 1 s 2 2 s 1 1 s 2 2 s 2 and so on for the remaining elements. When chemists write down
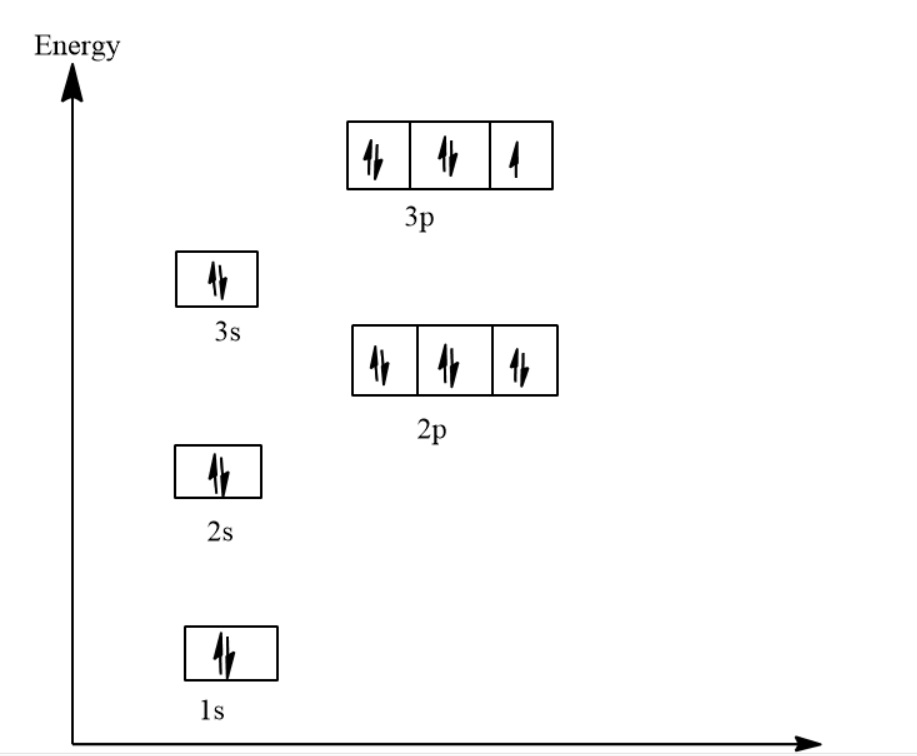
Source Image: bartleby.com
Download Image
Beryllium (Be). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of beryllium-9 (atomic numb Stock Photo – Alamy
Answered: Draw the electron configuration for a… | bartleby
Electron affinity The energy released when an electron is added to the neutral atom and a negative ion is formed. Electronegativity (Pauling scale) The tendency of an atom to attract electrons towards itself, expressed on a relative scale. First ionisation energy The minimum energy required to remove an electron from a neutral atom in its
Answered: Draw the electron configuration for a… | bartleby Electronic Configuration Of Neutral Beryllium He 2s2 Royalty Free SVG, Cliparts, Vectors, and Stock Illustration. Image 190384831.
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ).